+/-1 least significant digit at a minimum.
“I’m sorry frog, but you might actually weigh 0”. Little buddy noooo
I once met a person that never drank water, only soft drinks. It’s not the unhealthiness of this that disturbed me, but the fact they did it without the requisite paperwork.
Unlike those disorganised people I have a formal waiver. I primarily drink steam and crushed glaciers.
+/-1 least significant digit at a minimum.
“I’m sorry frog, but you might actually weigh 0”. Little buddy noooo
Absolutely amazing. Going to go for the offline port though, I don’t trust my save data to my browser.
N.B. Only worked in Chromium (not Firefox) for me. Could be due to addons though, not sure.


$BILLIONS
I mentally read this in the same voice I read $VARIABLE with.
Are red & blue lines under the pic are the calibrated references, whilst the car pics are not?


Every news website is covering it. I think I’ve spotted most of 10 articles around the place.
The law of well-marketed unreleased goods dictates that this vehicle is not going to meet any of the promises mentioned in the articles. I hope to be proven wrong, but just like video games: don’t pre-order, wait for it to come out and be reviewed.


That’s a CH340G, it has an in-built 3.3V regulator. But there is no external regulator on the board.
Maybe the chip is running off its internal 3.3V, but the board designers put a tie-up resistor on one of its pins to 5V, which results in the weird 3.9V. Dunno. Try attaching a 1K resistor between that pin a GND, see if that makes the problem disappear.


The 5.3V is from your computer, that’s not the fault of the USB UART.
3.2V is perfectly acceptable for a 3.3V rail.
The 3.9V is a bit weird. Can you post a photo of your USB UART board? Maybe the main chip has an inbuilt 3.3V regulator separate to the external one.
I swear that I read that white lead oxide is water soluble, thus happily sticks to your fingers and then gets on your food. I must be misremembering.
Maybe it was something about the solid lead object turning into an (oxide) powder that can then be easily ported as tiny particles on greasy hands? Hearsay science and safety information from me today :)
The fun thing about Pb is it’s relatively safe in pure form. Unfortunately the oxides that appear on its surface are water soluble and love entering our bodies.
Just looked this up, apparently I’m completely wrong. Maybe I was thinking about lipid compatibility? Not sure now.


Changing virtual desktops works for me, no patches needed. I have to use it often because of how many games don’t understand multiple monitors.


Technically they have some differences, but the biggest from a user’s perspective is how they are delivered and by whom. Wine is manually installed by you from your distro’s package repo. Proton is provided by steam when you install a windows game on a Linux steam instance. If one breaks then you complain to the relevant party.


GNOME 2 was fun and easy. It felt like they were trying to learn from the mistakes of Windows and Mac UIs.


Triangle is an amplifier and rectangle is a black box (“don’t worry what’s in here, we promise it’s not gremlins”).
I suspect that the box might be a biasing array for driving the two output transistors, but then I would also expect two wires to come out of it (one for each transistor) rather than a single combined wire.
Broadcom’s datasheet for their version of the part seems to be more akin to what I’m thinking:
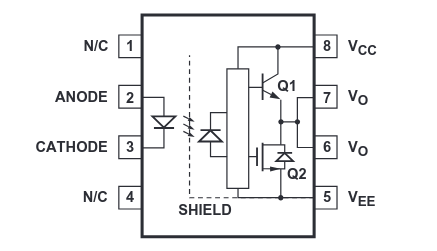
Could be either. You’d have to decap the chip to find out, the datasheet writers thought these details were not important.
I have no idea why two of the output pins are tied together. They’re not using many of the pins on this package so maybe they thought “why not”. I’ve also seen dual-optocouplers in this same 8 pin package where pins 6 & 7 are the outputs of the two separate couplers.


As a general rule I hold suspicion to any marketing that claims that using CO2 (or other products of burning) is environmental friendly. The products you get from burning fuels are supposed to be useless and of low value, otherwise they are not burning them efficiently.
To turn CO2 into potassium carbonate (pearl ash) you will need a lot of energy. Where they get this energy from is far more important than where they got the CO2 from. I would not be surprised if it is more environmentally friendly to make the pearl ash through a different process and ignore the CO2 rather than trying to convert the CO2 into pearl ash.
Background chemistry
Fuels are chemicals with a lot of potential chemical energy stored in them. They are generally considered (at a minimum) flammable or “reactive” in some way.
When we burn fuels we turn them into products with very little potential chemical energy, mainly CO2. You cannot burn CO2 and get energy out of it, it is a “stable” or “unreactive” chemical. It has very little chemical energy stored in it compared to the original fuel.
The difference in stored chemical energy between the fuel (eg methane CH4) and the products (eg CO2) is turned into heat and then electricity (via steam turbines). If your products are still reactive then you have not used them to their full potential and you will not get as much heat out as you could (not to mention improperly burned products tend to be toxic, eg carbon monoxide).
Now let’s look at potassium carbonate (K2CO3). It’s a somewhat reactive chemical, it’s not anywhere as stable as CO2, you can observe this by the fact it readily wants to react with other chemicals (caveman test: put it on your skin and it will sting). CO2 is very stable and does not want to do much (caveman test: put it on your skin and you won’t feel it).
To make K2CO3 from CO2 you will require energy input. Turning an unreactive chemical into a reactive one is a bit like the reverse of burning something. This energy will probably come from burning more coal or gas. I suspect it will require more coal/gas than making the CO2 did, so net overall you will probably be releasing more CO2 than you capture and turn into K2CO3.
Of course if they’re using renewal energy (solar) for this step then this could be a net positive.
My level of trust in the honesty of product packaging and marketing is pretty low and if they don’t mention it then they obviously don’t think it’s important. 🤷
EDIT: I’ll also add that “carbon capture” projects (things that claim to get rid of or make use of the CO2 from burning fuels) are universally disasters or scams.
EDIT2: I’ve taking some simplification liberties with the chemistry here. Technically CO2 isn’t completely stable, you can do stuff like make weak acids in water with it, but I do not believe anyone has found a way for that to usefully use up what we emit from burning fuels.
Perfect dark has a fan PC port that’s really good. I couldn’t stand it on console (low fps made me motionsick) but it was a hoot when I played it on PC. https://github.com/fgsfdsfgs/perfect_dark


I click on my “From” address and then select “Customize From Address…”. I can then type anything I want up there. It’s a little annoying when replying to an email chain with an alias, but not too many steps.


I was amazed that we transitioned from one GPU heavy bubble (Crypto) to another (LLM/AI). Whilst the hype for crypto imploded the use for the hardware sort of didn’t. I wonder if the next bubble with be the same, or if we get some refreshing variety to our money sinks?
Microsoft et al are subsidizing GenAI to an insane degree. […] prices shoot up for their customers and serve as a rough awakening to all the websites that integrated a crappy chatbot.
I’ve run some much simpler chatbots on just my desktop PC, so they will have some fallback (if they really choose to take it). Still it locks up my entire computer for a few second for each reply, so even a few hundred users per second peak would be an expensive service.
(Insert joke here about customers not noticing or caring about the difference between website chatbots built on big company services vs smaller ones, because they have exactly the same problems just in different hues.)


I’m confused. Sacholding?

Read-only, or the ability to edit filenames & upload files?
Read only: as per other answers here, basically any HTTP server. The easiest one I know would be darkhttpd, because it requires no config files and can be run without root.
Read write: I like WFM https://github.com/tenox7/wfm